How many bonds can Carbon form?
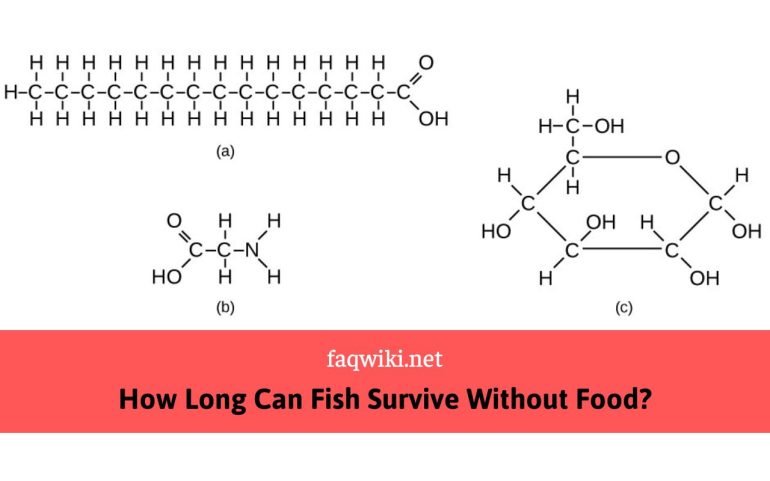
How many bonds can Carbon form? Carbon is one of the most abundant elements on Earth and is a fundamental building block of life. It has the unique ability to form an almost limitless number of compounds due to its four valence electrons, which allows it to bond with other atoms in a variety of ways. In this article, we will explore the basics of carbon bonding, including how many bonds carbon can form, the types of bonds it can form, and the various factors that influence carbon bonding.
Carbon Bonding Basics
Chemical bonding occurs when two or more atoms combine to form a molecule. Atoms can form chemical bonds in several ways, including ionic bonding, covalent bonding, and metallic bonding. However, carbon primarily forms covalent bonds, which occur when two or more atoms share electrons in order to achieve a more stable electron configuration.
Covalent bonds are essential to organic chemistry, which is the study of compounds containing carbon. Carbon can form up to four covalent bonds with other atoms due to having four valence electrons. This ability to form multiple covalent bonds allows carbon to create a vast array of organic molecules with unique properties and functions.
Carbon’s Valence Electrons
Valence electrons are the outermost electrons in an atom’s electron shell, and they are the electrons involved in chemical bonding. Carbon has four valence electrons, which means it has the ability to form up to four covalent bonds with other atoms. The number of valence electrons an atom has determines its chemical behavior and its ability to bond with other atoms.
Four Covalent Bonds of Carbon
Carbon can form up to four covalent bonds, which can be single, double, or triple bonds. A single covalent bond is formed when two atoms share one pair of electrons. In a double covalent bond, two atoms share two pairs of electrons, and in a triple covalent bond, two atoms share three pairs of electrons.
The number and type of covalent bonds that carbon forms depend on the number and types of atoms it bonds with. For example, when carbon bonds with four hydrogen atoms, it forms four single covalent bonds. This molecule is called methane, and it is the simplest organic molecule.
Carbon can also form double and triple covalent bonds with other atoms. For example, when carbon bonds with oxygen, it can form a double covalent bond to create carbon dioxide. Carbon can also form triple covalent bonds with other carbon atoms, as in the case of acetylene.
Hybridization and Carbon Bonding
Hybridization is the process by which atomic orbitals combine to form new hybrid orbitals, which can then participate in covalent bonding. Carbon undergoes hybridization to form its four covalent bonds. There exist three types of hybridization, namely sp, sp2, and sp3.
In sp hybridization, one s orbital and one p orbital combine to form two hybrid orbitals, each oriented at a 180-degree angle from each other. This type of hybridization occurs in molecules such as acetylene, where carbon forms a triple bond with another carbon atom.
In sp2 hybridization, one s orbital and two p orbitals combine to form three hybrid orbitals, each oriented at a 120-degree angle from each other. This type of hybridization occurs in molecules such as ethylene, where carbon forms a double bond with another carbon atom.
In sp3 hybridization, one s orbital and three p orbitals combine to form four hybrid orbitals, each oriented at a 109.5-degree angle from each other. This type of hybridization occurs in molecules such as methane, where carbon forms four single covalent bonds with hydrogen atoms.
The type of hybridization that carbon undergoes depends on the number and types of atoms it bonds with. For example, if carbon bonds with three hydrogen atoms and one nitrogen atom, it undergoes sp3 hybridization to form four single covalent bonds.
Factors That Affect Carbon Bonding
Several factors can influence how carbon bonds with other atoms, including the types of atoms it bonds with, the bond lengths and strengths, and the molecular geometry of the resulting molecule.
The types of atoms carbon bonds with can affect the type and number of covalent bonds it forms. For example, carbon tends to form double and triple bonds with atoms such as oxygen and nitrogen, which are more electronegative than carbon.
Bond lengths and strengths can also affect carbon bonding. A shorter bond length indicates a stronger bond, and a longer bond length indicates a weaker bond. The strength of a covalent bond between carbon and another atom depends on factors such as the types of atoms involved and the bond length.
Finally, the molecular geometry of the resulting molecule can also affect carbon bonding. The term “molecular geometry” pertains to how atoms are arranged in a molecule in three dimensions. The arrangement of atoms can affect the polarity of the molecule and its ability to interact with other molecules.
Applications of Carbon Bonding
Carbon bonding has numerous applications in fields such as medicine, agriculture, and materials science. Organic molecules containing carbon and other elements are essential to life, and they play a vital role in many biological processes. For example, enzymes are organic molecules that contain carbon and are essential to many biochemical reactions.
Carbon bonding is also important in agriculture, where organic molecules such as fertilizers and pesticides are used to enhance crop growth and protect plants from pests and diseases. In materials science, carbon bonding is used to create materials with unique properties, such as carbon fibers, which are lightweight and strong and have numerous applications in aerospace and automotive industries.
Read more science articles here!
Conclusion
Carbon’s unique ability to form multiple covalent bonds with other atoms makes it an essential element in organic chemistry and the study of life. Carbon bonding is a fundamental concept in chemistry, and understanding how it works is essential to understanding many aspects of science and technology.
In conclusion, carbon’s ability to form an almost limitless number of compounds has revolutionized our world, from the molecules that make up our bodies to the materials we use every day. By understanding the basics of carbon bonding, we can unlock the boundless potential of this essential element.
FAQs
Q: How many covalent bonds can carbon form?
A: One carbon atom can create up to four covalent bonds with other atoms.
Q: What types of covalent bonds can carbon form?
A: Carbon can form single, double, and triple covalent bonds.
Q: What factors can influence carbon bonding?
A: The types of atoms carbon bonds with, bond lengths and strengths, and molecular geometry can all influence carbon bonding.
Q: What are some applications of carbon bonding?
A: Carbon bonding is essential to life, agriculture, and materials science, and has numerous applications in fields such as medicine, aerospace, and automotive industries.
